Molecular Level
 Determining the identity and concentration of molecules in a gas mixture is often essential in industrial, environmental, research, or workplace settings. There are various sensors available, such as infrared, electrochemical, and catalytic sensors, to detect the quantity of specific gases. These sensors are usually very sensitive to specific molecules, but due to their technical limitations, they are not suitable for detecting other components. But what if it's unclear which gas is present in the space?
Determining the identity and concentration of molecules in a gas mixture is often essential in industrial, environmental, research, or workplace settings. There are various sensors available, such as infrared, electrochemical, and catalytic sensors, to detect the quantity of specific gases. These sensors are usually very sensitive to specific molecules, but due to their technical limitations, they are not suitable for detecting other components. But what if it's unclear which gas is present in the space?
How Is Identity Determined?
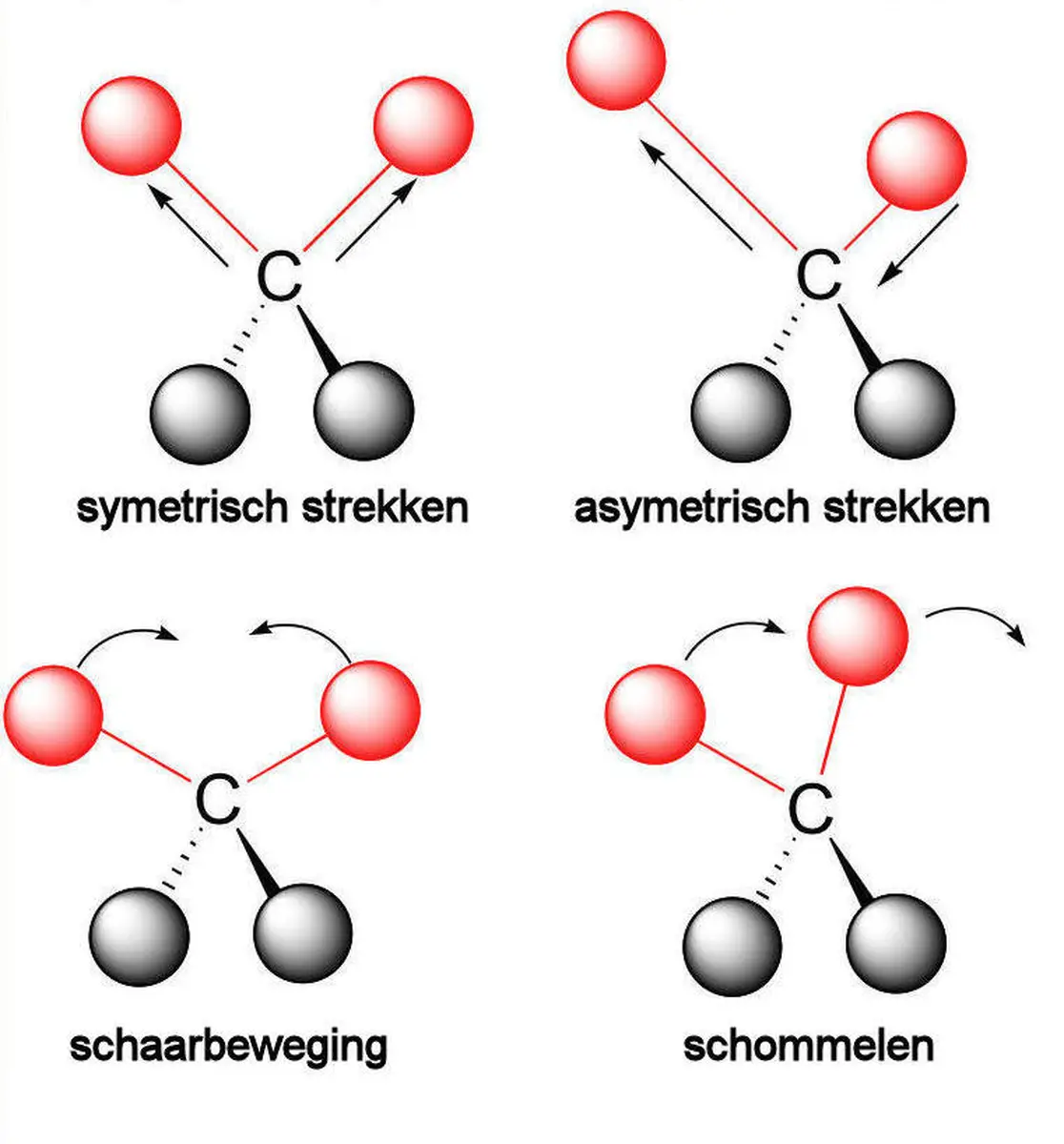
One of the methods to identify an unknown gas is Fourier Transform Infrared (FTIR) spectroscopy. This technique is based on the interaction between molecules and light. A simple model of a molecular bond can be visualized as two masses connected by a spring, where the masses represent the two atoms and the spring (with spring constant k) represents the bond. The natural frequency is unique to each molecular bond. Of course, this is a simplified model. In reality, molecular bonds exhibit motions such as symmetric stretching, asymmetric stretching, scissoring, and rocking when excited by the correct energy/frequency. Although these vibrations differ from the basic spring model, each has a unique frequency. These frequencies can be used to create a molecular "fingerprint" of the gas using an FTIR spectrophotometer.
How Does an FTIR Spectrophotometer Work?
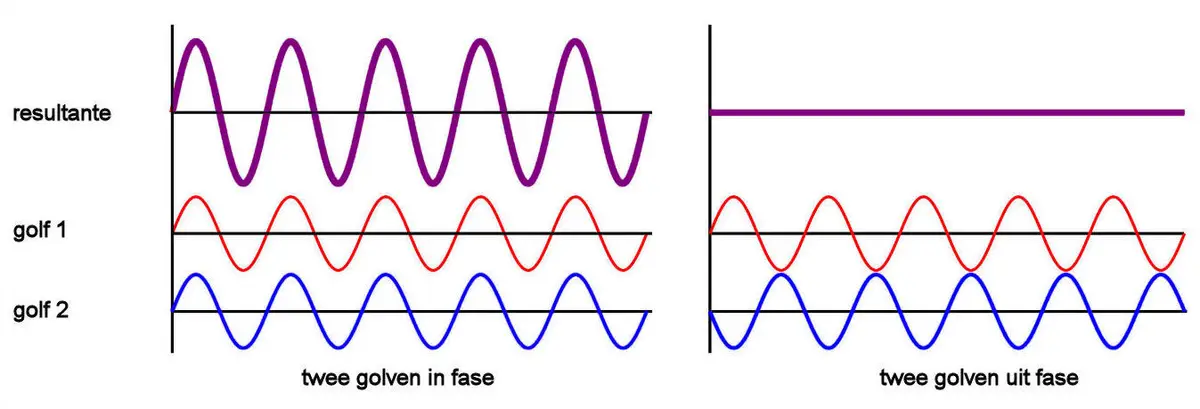 Light from nearly the entire IR spectrum is simultaneously directed at a semi-transparent mirror. Half of the light is transmitted, while the other half is reflected at a 90-degree angle. The light is then reflected off mirror 1 or a movable mirror 2. The position of mirror 2 is precisely measured using a laser. The light travels two different path lengths, d1 and d2. After reflection, the beams return to the semi-transparent mirror and interfere with each other. Interference occurs when two or more light waves overlap, forming a pattern. If the path difference d = 2.(d1 - d2) is zero or a multiple of the wavelength, constructive interference occurs. If the difference is half a wavelength, destructive interference occurs. The resulting interferogram plots the mirror position against the measured intensity at the detector.
Light from nearly the entire IR spectrum is simultaneously directed at a semi-transparent mirror. Half of the light is transmitted, while the other half is reflected at a 90-degree angle. The light is then reflected off mirror 1 or a movable mirror 2. The position of mirror 2 is precisely measured using a laser. The light travels two different path lengths, d1 and d2. After reflection, the beams return to the semi-transparent mirror and interfere with each other. Interference occurs when two or more light waves overlap, forming a pattern. If the path difference d = 2.(d1 - d2) is zero or a multiple of the wavelength, constructive interference occurs. If the difference is half a wavelength, destructive interference occurs. The resulting interferogram plots the mirror position against the measured intensity at the detector.
Software
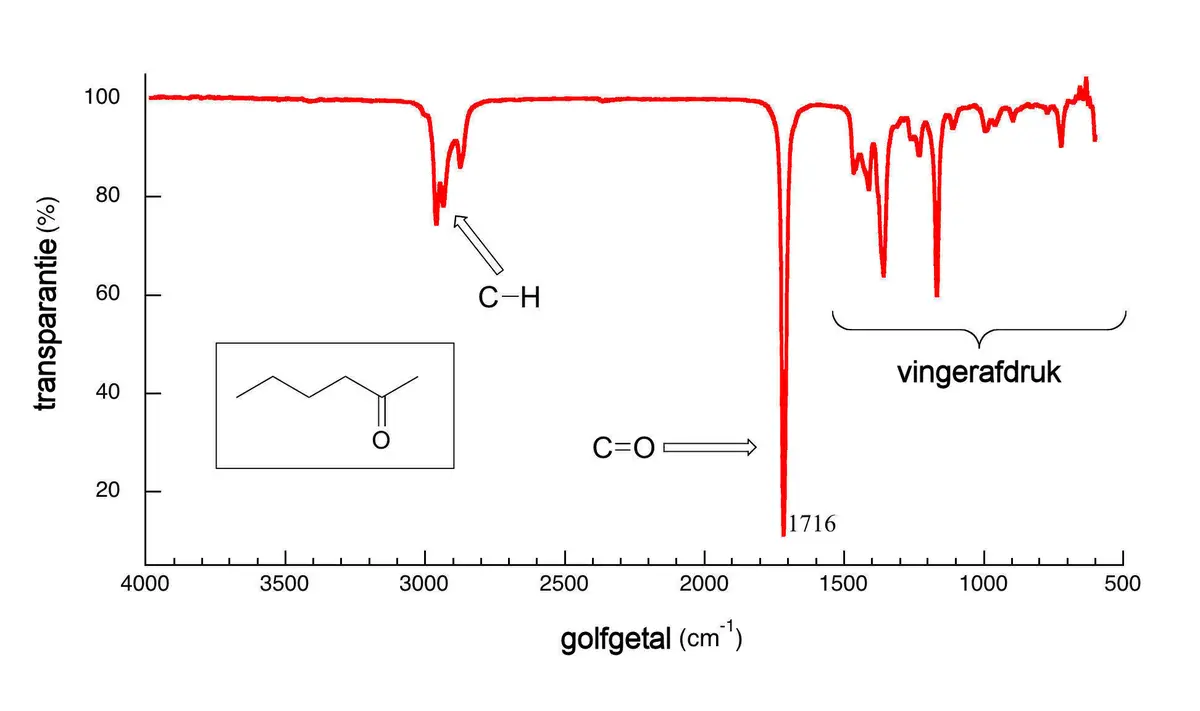 The analyzer software uses a Fourier transformation to convert the interferogram into a spectrum showing the wavenumber [cm-1] versus transmission. 100% transmission means all transmitted light reaches the detector. In the above FTIR spectrum, specific bonds such as C-H and C=O are clearly visible. These patterns in the spectrum are like the ridges of a fingerprint. By comparing the transmission spectrum to a library of reference spectra, the identity of the gas (or culprit) can be determined with a certain degree of certainty. Depending on the software, it is also possible to analyze multiple components simultaneously. For example, the Calcmet™ software from Gasmet FTIR analyzers can simultaneously analyze up to 50 gases. FTIR analyzers are available in portable and stationary configurations (wall-mounted or 19” rack).
The analyzer software uses a Fourier transformation to convert the interferogram into a spectrum showing the wavenumber [cm-1] versus transmission. 100% transmission means all transmitted light reaches the detector. In the above FTIR spectrum, specific bonds such as C-H and C=O are clearly visible. These patterns in the spectrum are like the ridges of a fingerprint. By comparing the transmission spectrum to a library of reference spectra, the identity of the gas (or culprit) can be determined with a certain degree of certainty. Depending on the software, it is also possible to analyze multiple components simultaneously. For example, the Calcmet™ software from Gasmet FTIR analyzers can simultaneously analyze up to 50 gases. FTIR analyzers are available in portable and stationary configurations (wall-mounted or 19” rack).
