Development of Sensor Arrays
Importance of Gas Detection
The detection of gases in urban and industrial environments is important. It ensures that people can work in a healthy and safe environment. For almost every gas, there is a sensor that can detect it and determine its concentration. In an ideal world, a sensor's response would depend solely on the concentration of the specific gas component. In this case, the detected signal SA by sensor A for component A would look like:S_A = k_AA.[A] [1]Sensor Response Mechanism
Where SA is the measured signal by sensor A, k_AA is the response factor of sensor A to molecule A, and [A] is the concentration of molecule A.
Linear Relationships and Real-World Challenges
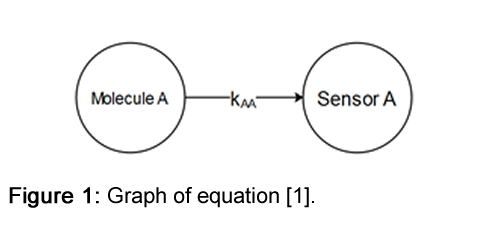 See Figure 1.
See Figure 1.
Most sensors developed show a linear relationship between the substance concentration and signal strength; if not, the signal is linearized. In reality, sensor responses are not ideal, as they can react with various components and are influenced by changes in temperature, humidity, and pressure. The influence of temperature, humidity, and pressure on the measured signal is beyond the scope of this article.
Sensor Cross-Sensitivity
The response of a sensor to different molecules is called cross-sensitivity. For example, if we have two molecules A and B, the response of sensor A can be described as:
S_A = k_AA.[A] + k_BA.[B] [2]The graph of equation [2] looks like:
See Figure 2.
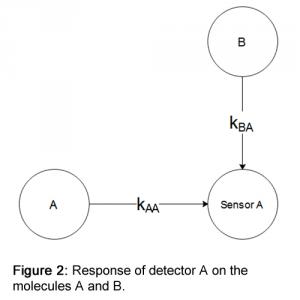 In the ideal situation, kBA—the response of sensor A to molecule B—is zero. If the value of kBA is not zero, there is a problem. It is no longer clear whether the sensor is responding to molecule A or B. If we have no information other than the signal strength coming from the sensor, the solution to [2] is a line. For most applications, this is not an acceptable solution. To overcome this problem, Ravebo B.V. is developing a sensor array. The main idea behind the sensor array is to develop a system that can use relatively inexpensive sensors while still measuring the concentrations of various components with high precision. The sensor array consists of a number of sensors equal to the number of available gases and a matrix K that compensates for all known cross-interferences.
In the ideal situation, kBA—the response of sensor A to molecule B—is zero. If the value of kBA is not zero, there is a problem. It is no longer clear whether the sensor is responding to molecule A or B. If we have no information other than the signal strength coming from the sensor, the solution to [2] is a line. For most applications, this is not an acceptable solution. To overcome this problem, Ravebo B.V. is developing a sensor array. The main idea behind the sensor array is to develop a system that can use relatively inexpensive sensors while still measuring the concentrations of various components with high precision. The sensor array consists of a number of sensors equal to the number of available gases and a matrix K that compensates for all known cross-interferences.
Solution via Sensor Arrays
The matrix calculation is as follows:
S=Kc [3]Where S is the sensor response vector, K is the cross-interference matrix, and c is the concentration vector.
Matrix Calculations for Sensors
If the matrix K has an inverse K_inv, we can solve equation [3] for c.
K_inv.S = K_inv.K.c = c [4]Thus, the main problem of finding the true concentrations c is reduced to finding the matrix K and its inverse.
Procedure for Determining Matrix K
To determine matrix K, the following procedure can be used.
Determine the gases present in the gas matrix. In our example, we use gases A, B, and C. Creating a graph is usually a good starting point to visualize the various interactions.
See Figure 3.
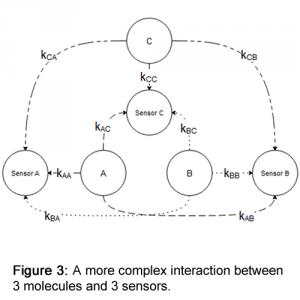 Use different calibration gases to determine the response factors of each sensor to the applied gases and assess their linearity. From these tests, the various k-values can be calculated.
Use different calibration gases to determine the response factors of each sensor to the applied gases and assess their linearity. From these tests, the various k-values can be calculated.
Create the K matrix with the different k-values. In our example, it would take the form:
K = [[k_AA, k_AB, k_AC], [k_BA, k_BB, k_BC], [k_CA, k_CB, k_CC]]Determine whether the determinant of matrix K is not zero. If the determinant is zero, matrix K is not invertible.
Use Gaussian elimination or another procedure to determine K_inv.
After the above procedure, it is now relatively easy to calculate the actual concentrations c instead of the measured concentration S.
