FID Operating Principle
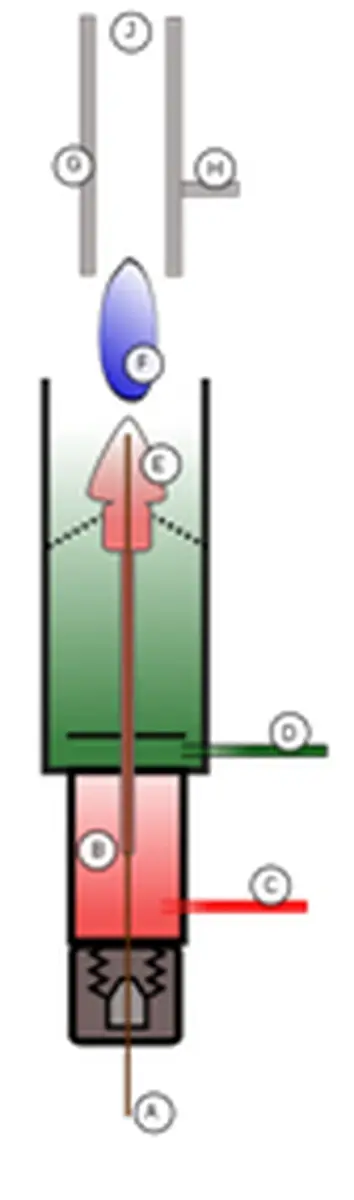 FID stands for Flame Ionization Detector and utilizes the fact that during the combustion of hydrocarbons, ions and electrons are produced as byproducts. In the FID analyzer, the sample gas (A) is mixed with the fuel gas (C) inside the oven (B). This mixture is then combined with oxygen (D), resulting in the formation of CHO+ ions. These ions are repelled by the positively charged nozzle (E) and attracted toward the negatively charged collector plates (G). When an ion hits the collector plate, a current is generated, which is measured using a highly sensitive ammeter. The magnitude of this current correlates with the number of carbon atoms in the hydrocarbons present in the sample. The current is integrated over time using an electronic circuit, and the resulting area under the curve represents the concentration.
FID stands for Flame Ionization Detector and utilizes the fact that during the combustion of hydrocarbons, ions and electrons are produced as byproducts. In the FID analyzer, the sample gas (A) is mixed with the fuel gas (C) inside the oven (B). This mixture is then combined with oxygen (D), resulting in the formation of CHO+ ions. These ions are repelled by the positively charged nozzle (E) and attracted toward the negatively charged collector plates (G). When an ion hits the collector plate, a current is generated, which is measured using a highly sensitive ammeter. The magnitude of this current correlates with the number of carbon atoms in the hydrocarbons present in the sample. The current is integrated over time using an electronic circuit, and the resulting area under the curve represents the concentration.
