Conditioning for Online Dust Measurements in Emissions, Processes, and Environments
 The biggest challenges for stationary dust measurements using an optical measurement principle lie in the conditions of the sampled gas stream (or ambient air). For many processes, the moisture content is too high to directly derive dust content optically. Additionally, in process streams prior to any purification treatment, the particle load is often too high for this measurement principle. When conditioning the gas, it is essential to minimize unnecessary losses. We mainly work with three principles: heating, dilution, or removing water using a permeation dryer (Nafion® membrane).
The biggest challenges for stationary dust measurements using an optical measurement principle lie in the conditions of the sampled gas stream (or ambient air). For many processes, the moisture content is too high to directly derive dust content optically. Additionally, in process streams prior to any purification treatment, the particle load is often too high for this measurement principle. When conditioning the gas, it is essential to minimize unnecessary losses. We mainly work with three principles: heating, dilution, or removing water using a permeation dryer (Nafion® membrane).
Gas Stream Dilution
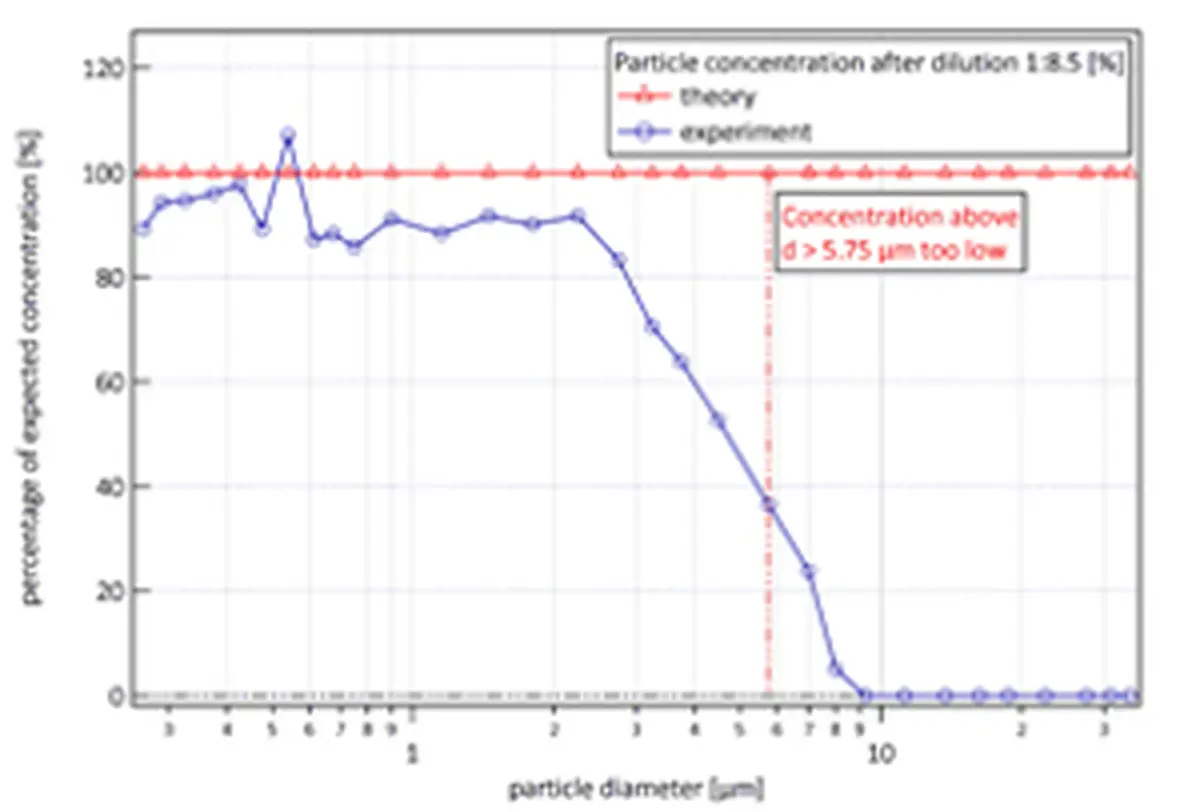 The first problem occurs when the particle count is too high for the particle analyzer. It then becomes impossible to distinguish between individual particles. To avoid this, dilution can be applied to significantly reduce the particle concentration. In the example below, the airflow is diluted with particle-free air at a ratio of 1:8.5. However, this introduces side effects that must be corrected for. There is a disproportionate loss of particles of different diameters, caused by collisions between the particles. Larger particles are more likely to collide with other large particles than small ones are with other small ones.
The first problem occurs when the particle count is too high for the particle analyzer. It then becomes impossible to distinguish between individual particles. To avoid this, dilution can be applied to significantly reduce the particle concentration. In the example below, the airflow is diluted with particle-free air at a ratio of 1:8.5. However, this introduces side effects that must be corrected for. There is a disproportionate loss of particles of different diameters, caused by collisions between the particles. Larger particles are more likely to collide with other large particles than small ones are with other small ones.
Heating of Gas Streams
A second conditioning step is heating the gas. This can be used when condensation is expected or to investigate the composition of particulate matter. When heating is applied, the aerosol formation process is reversed. Depending on the chosen temperature, certain organic components may break down and disappear in real-time measurements. If the particulate matter consists of both organic and inorganic fractions, this method can help provide insight into its composition.
Chemical Drying
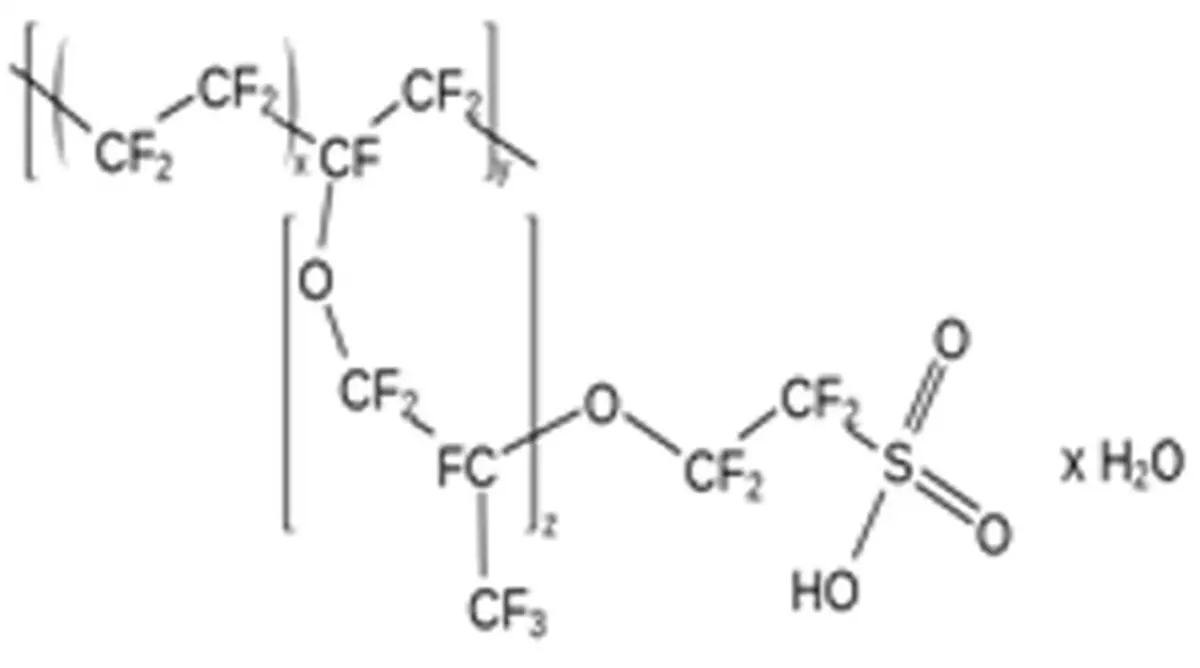 A way to remove water from the gas stream without particle loss is by using Nafion tubing. This is a Teflon variant modified with sulfonic acid groups. Water is attracted by these groups and diffuses through them to the side with the lowest water concentration.
A way to remove water from the gas stream without particle loss is by using Nafion tubing. This is a Teflon variant modified with sulfonic acid groups. Water is attracted by these groups and diffuses through them to the side with the lowest water concentration.
Fine dust is often detected in environments with relatively dry air and low particle counts. Fine dust is divided into two fractions:
Primary Fine Dust:
Created by friction. For example, during the grinding of materials in the feed or chemical industries. Another example is wind erosion that scrapes particles along buildings or rocks. The combustion of fossil fuels like coal, oil, and gas also releases primary fine dust. This dust is directly emitted into the air as particles. 
Secondary Fine Dust:
Forms when molecules of acidifying substances bind to salts. Think of substances such as nitrogen oxides (NOx), sulfur dioxide (SO2), and ammonia (NH3). These can also attach to primary fine dust particles.
Appropriate Conditioning
Each conditioning step has its pros and cons. For every process application, we advise suitable conditioning—if possible. Considerations include: loss of volatile components in aerosol form when the temperature increases, misattributing mass to water droplets, particle concentration, refractive index, and density of the components. If the focus is on coarser particles (>1 micron), isokinetic sampling is essential. Smaller particles distribute more evenly, and non-isokinetic deviations are usually minor. For discontinuous measurements, a portable monitor with proper conditioning in a suitable enclosure may be a good solution. For continuous, long-term monitoring (months or 365 days a year), we recommend systems with durable pump setups suitable for continuous operation. If direct measurement of dust in a duct is not possible using a triboelectric or optical method, the challenge lies in applying the correct conditioning and selecting the most suitable measurement principle for the desired online dust determination.
